ILSIPP POSITION STATEMENT
Percutaneous Peripheral Nerve Stimulation (PNS)
March 1, 2024
Position Statement
The ILLINOIS Society of Interventional Pain Physicians (ILSIPP) strongly supports the use of Peripheral Nerve Stimulation (PNS) for those patients with intractable neuropathic pain who have failed previous conservative and/or invasive modalities. Patients in this category have no credible alternatives. Based upon review of the body of peer reviewed published evidence, approval by the United States Food and Drug Administration (FDA), real world experience, and long term patient outcomes, ILSIPPrecommends qualified physicians consider the use of this modality based upon clinical need and presentation. Because of proven safety and durable effectiveness, PNS systems are within the standard of care for their indicated use.
Based upon the body of peer-reviewed published evidence, FDA approval, recognition under a Medicare National Coverage Determination (160.7), and robust support from esteemed entities like the National Institutes of Health and the Department of Defense, ILSIPPfurther recommends policymakers and payers enable timely access to PNS when prescribed by a qualified physician who has used his or her best medical judgement in caring for those patients with focal neuropathic pain syndromes.
Background
Percutaneous Peripheral Nerve Stimulation (PNS) employs electrical pulses through small electrodes percutaneously implanted near targeted peripheral nerves, offering a non-opioid alternative for chronic pain since FDA approval in 2016. Temporary trial stimulation can be followed by permanent implantation when indicated. ILSIPPadvocates for the use of PNS systems in managing chronic pain that persists after two or more conservative treatments have failed. The following devices hold FDA clearance for temporary and/or permanent implantation:
- Freedom PNS System – Curonix
- Nalu PNS System – Nalu Medical
- SPRINT PNS System – SPR Therapeutics
- StimRouter System – Bioventis
The first theme that must be addressed is the importance of providing patients who can benefit from PNS devices with access to these devices. PNS devices that have met rigorous requirements such as pre–market approval should be made available to patients who can benefit. Patients who have exhausted all conservative therapies and still suffer from pain should have access to these devices as a viable alternative.
Next, the safety of PNS devices must be acknowledged. PNS devices have an established historical profile for safety (2,4). Additionally, it is important to note that PNS devices are not novel technologies, and their use has been validated in multiple clinical studies (2,3,4,5,6).
Finally, the application of neuromodulation, including PNS, has been shown to be a cost-effective option in the treatment of chronic pain compared to other treatment options. One study demonstrated reductions in physician office visits, nerve blocks, radiologic imaging, emergency department visits, hospitalizations, and surgical procedures. This translated to a net annual savings of c. $30,221 and a savings of $93,685 over the 3-year implant duration. The large reduction in healthcare utilization following spinal cord/peripheral nerve stimulation implantation resulted in a net per patient per year cost savings of approximately $17,903 (1).
Over 30 peer reviewed publications, including randomized controlled trials (2,3), endorse the efficacy of PNS systems in managing pain of the shoulder, back, knee, foot, and other conditions including phantom limb pain. Clinical studies consistently demonstrate positive outcomes across neuropathic syndromes, further validating PNS as standard of care. The most recent randomized controlled study (RCT) across 14 pain management centers (COMFORT PNS RCT) demonstrated an 87% responder rate (>50% pain reduction) at one year (2,3). Categorizing this treatment as “experimental and investigational” is grossly inaccurate.
Conclusion and Recommendations
ILSIPPstrongly advocates for the utilization of Percutaneous Peripheral Nerve Stimulation (PNS) by qualified, trained physicians for patients with focal neuropathic pain syndromes who have failed > 6 months of conservative therapy. The body of published evidence, long term outcomes demonstrating durable treatment effect, low complication rate, as well as cost effectiveness makes this an essential tool in the management of refractory neuropathic pain syndromes. Additionally, with the support of Medicare National Coverage Determinations and the National Institutes of Health, it has become the standard of care within the Pain Management community. Policymakers and payers are strongly encouraged to enable timely access to FDA approved technologies when deemed medically necessary and indicated.
References
- Mekhail, et. al. “Cost Benefit Analysis of Neurostimulation for Chronic Pain”. The Clinical Journal of Pain. 2004. Nov-Dec; 20(6): 462-468.
- Engle M. et. al. “A Report on the Interim Long-Term Pain Outcomes from the COMFORT Peripheral Nerve Stimulation RCT”. Presented at 2024 NANS Annual Meeting.
- Hatheway J. et. al. “Holistic Outcomes from the COMFORT PNS RCT”. Presented at 2024 NANS Annual Meeting.
- Helm S. et. al. “Peripheral Nerve Stimulation for Chronic Pain: A Systematic Review of Effectiveness and Safety”. Pain and Therapy. 2021. Dec;10(2): 985-1002.
- Deer TR, et. al. “A Systematic Literature Review of Peripheral Nerve Stimulation Therapies for the Treatment of Pain”. Pain Medicine. 2020. August; 21(8): 1590-1603.
- Strand N. et. al. “Evidence -Based Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Nerve Stimulation in the Treatment of Chronic Pain”. Journal of Pain Research. 2022. August 23; 15: 2483-2504.
Position Statement on Treatment Alternatives for Patients with
Lumbar Spinal Stenosis
July 15, 2021
The ILLINOIS Society of Interventional Pain Physicians supports the use of the SuperionTM Indirect Decompression Systems (IDS) as an option for the treatment of lumbar stenosis patients as it augments the prevailing solutions and reaches additional patients who would otherwise be left untreated.
Lumbar spinal stenosis (LSS) is a condition in which the spinal canal becomes increasing narrowed from degenerative changes. Patients with LSS may experience symptoms of neurogenic claudication, including pain or discomfort that radiates to their lower leg, thigh, and/or buttocks while walking. Patients with more pronounced LSS report symptoms of develop lower extremity of weakness, muscle cramping, numbness, and imbalance. LSS is a debilitating, degenerative condition that worsens over time when left untreated. 1 Because of the dynamic nature of LSS, the pain is worsened when walking or standing, and relieved when bending forward, sitting or in the forward flexing position.
Offering an alternative option for the symptomatic LSS patient, which restores functional capacity and alleviates back and leg pain as well as other associated symptoms such as cramping, numbness and weakness without the reliance on medication is a much-needed therapy option.
ILLINOIS Society of Interventional Pain Physicians support the use of the Superion TM Indirect Decompression Systems (IDS) as an option for the treatment of lumbar stenosis patients as it augments the prevailing solutions and reaches additional patients who would otherwise be left untreated.
Interspinous spacer decompression using the Superion device offers a less invasive procedure for patients who fail conservative treatment before traditional more invasive surgery. It serves as an extension blocker, which in turn relieves pressure on the affected nerves, helping to minimize the clinical impact of dynamic spinal stenosis while fully preserving the patient’s nascent architecture and anatomy. 2 Superion’s mechanism of action addresses the root cause of stenosis in moderately stenosed patients rather simply palliating the symptoms. Its effectiveness mirrors those of the most invasive procedures, without exposing patients to longer recovery times, complication risks. It provides an option to those too frail to undergo more invasive procedures because it preserves the spinal anatomy for the patient as well as the surgeon should a future decompression become necessary.
PROCEDURE
The Superion ISS may be implanted at one or two adjacent lumbar levels in patients whom treatment is indicated and at no more than two levels, from L1 to L5. The device is inserted through a cannula about the size of a dime and thus requires no surgical dissection of the spinal musculature. The procedure is performed in an outpatient setting or ASC. The device may be implanted by an interventional pain specialist or a surgeon.
FDA INDICATION AND LABELING
The Superion ISS received FDA approval in 2015 based on a prospective, multi-site randomized clinical trial of Superion (n=190 patients) versus XSTOP (n=201 patients) with 3 year follow up.
“This device is indicated to treat skeletally mature patients suffering from pain, numbness, and/or cramping in the legs (neurogenic intermittent claudication) secondary to a diagnosis of moderate degenerative lumbar spinal stenosis….
- Impaired physical function who experience relief in flexion from symptoms of leg/buttock/groin pain
- numbness and/or cramping with or without back pain
- patients have undergone at least 6 months of non-operative treatment
CLINICAL EVIDENCE
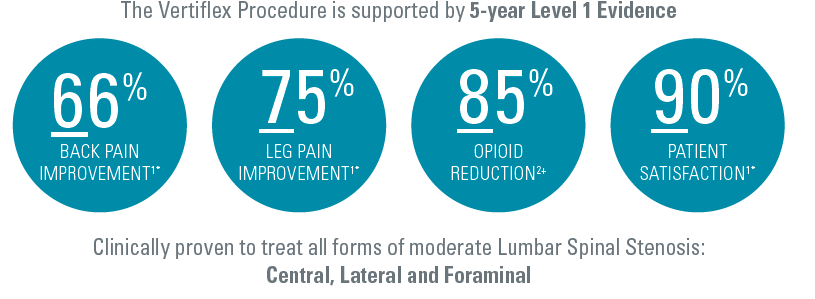
The Vertiflex procedure is supported by 5 Year Level 1 Evidence. At 3 years, Superion (63/120, 52.5%) vs X STOP (49/129, 38%), (P=.023) achieved the desired endpoint and maintained improvement in back and leg pain.
- Success rate > 80% for each component of the primary endpoint in the Superion Group. (Range:81%-91%).
- NOTE: Primary composite endpoint: Improvement in two of three domains of the Zurich Claudication Questionnaire, no reoperations at the index level, no major implant-or procedure related complications, and no clinically significant confounding treatments.
Nunley (2018) reported on the pattern of opioid use in this pivotal study. At baseline, Nunley (2018) reported 50% (94 of 190) patients were using opioid medication. The study showed a sharp decrease in opioid utilization from 25.2% (41 of 163) at 12 months to 13.3% (20 of 150) at 24 months to 7.5% (8 of 107) at 60 months. 85% reduction in the proportion of patients using opioids compared to baseline at 5 years.
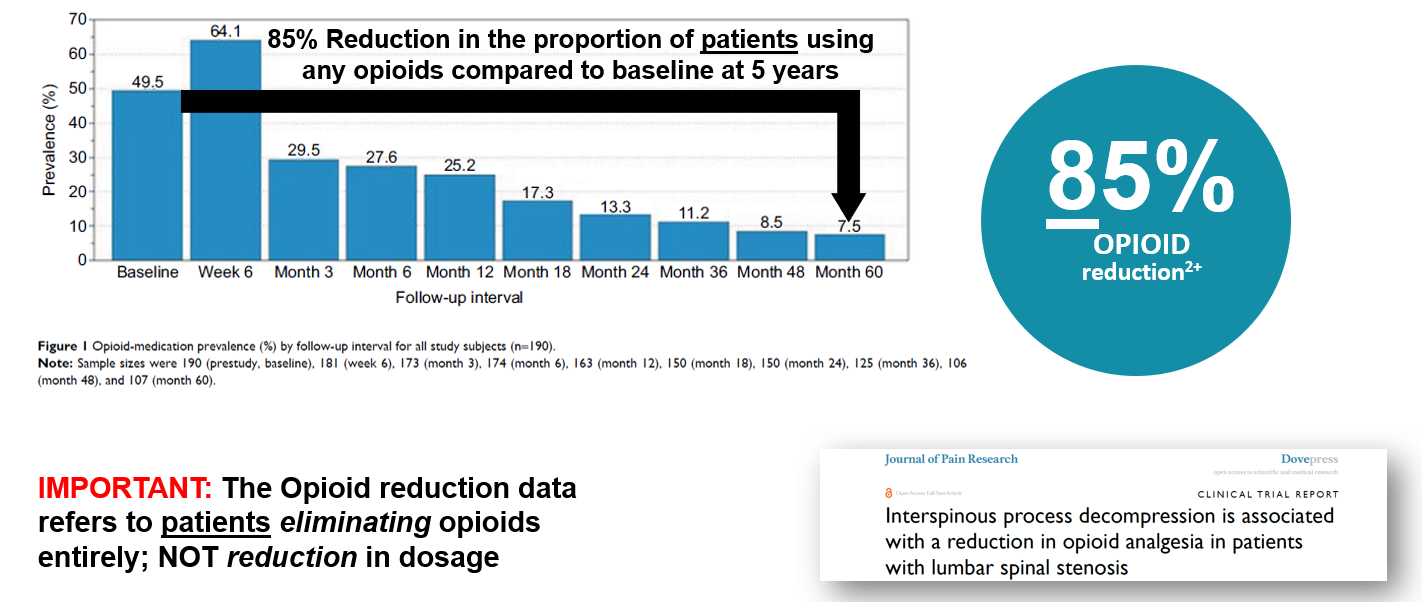
Three, four and five year follow up studies of Superion patients demonstrate solid outcomes.
- At 5 years post-procedure, there were statistically significant improvements in clinical outcomes (symptom severity and physical function) and severity leg and back pain according to data from a randomized, controlled FDA noninferiority trial.
- Parker et al. investigated the cost effectiveness of conservative care, laminectomy, and Superion. Although conservative care has the lowest costs ($10,540 USD) it was less effective. On average, the surgeries costs $3400 more per patient than the conservative strategy, with greater QALYs gained of 0.21 to 0.22
- Early results from an ongoing real-world registry following commercial patients who underwent the Vertiflex Procedure (n=1,523 at 6 mos., 423 at 12 mos.) show 75% of patients enjoying a clinically meaningful reduction in leg pain, 74% report significant functional improvement, an 80% patient satisfaction rate at 12 months post-procedure, and a low (5%) reoperation rate.
SOURCES:
- Nunley PD, Patel VV, Orndorff DG, et al. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017; 12: 1409 – 1417. Doi: 10.2147/cia.s143503.
- Vertiflex Superion Interspinous Spacer: PMA No. P140004. https://www.vertiflex.com/wp-content/uploads/2016/09/Superion-PMA-Summary.pdf. Accessed February 26, 2021
- FDA P140004 SSED
- Nunley PD, Patel VV, Orndorff DG, et al. Interspinous process decompression improves quality of life in patients with lumbar spinal stenosis. Minim Invasive Surg. 2018; 2018: 1035954. Doi: 10.1155/2018/1035954.
- Nunley PD, Patel VV, Orndorff DG, et al. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017; 12: 1409 – 1417. Doi: 10.2147/cia.s143503.
- Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: conservative care, laminectomy, and the Superion interspinous spacer. Int J Spine Surg. 2015; 9. Doi: 10.14444/2028.
- Deer T, et al. The MIST Guidelines: The Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2018; doi: 10.1111/papr.12744
ILSIPP POSITION STATEMENT
Indirect Decompression Systems Without Fusion
Indicated for Patient Selection for Moderate Stenosis
August 2021
ILSIPP Position Statement
Our Society supports the use of indirect decompression systems, also known as interspinous spacers or interspinous process distraction systems without fusion for patients diagnosed with moderate stenosis. Patients afflicted with the condition do not have credible alternatives, albeit surgical or conservative[i]. Based upon the review of the body of peer-reviewed published evidence, approval by the United States Food and Drug Administration (FDA), real world experience and long-term patient outcomes, ILSIPP recommends qualified physicians consider use of these minimally invasive interventions and inform patients in their treatment plans based upon the clinical need and presentation. Because of proven safety and durable effectiveness, standalone indirect decompression systems are within the clinical community’s standard of care for their indicated use. At the time of this publishing, the Superion indirect decompression system is the only FDA approved Indirect Decompression System without fusion marketed in the US.
Based upon the body of Level I-IV peer-reviewed published evidence, FDA approval, and demonstration of cost-effectiveness, ILSIPP further recommends policymakers and payers enable timely access to indirect decompression systems when prescribed by a qualified physician who has used his or her best medical judgement for care most suitable to the individual diagnosed with moderate lumbar spinal stenosis.
Moderate Stenosis: Burden of Disease
Spinal stenosis refers to a a narrowing of the spnal column or spinal anatomy in the areas of the central canal, lateral recess, and/or neural foramina. Stenosis may be congenital, but more likely degenerative in origin. Lumbar spinal stenosis affects more than 200,000 people in the United States and is considered the most common reason for spinal surgery in patients >65 years[ii].
In a claims-bases analysis, Parenteau et al (2021) reported the prevalence of a stenosis diagnosis over the age of 65 was >5% of the U.S. Medicare population, with women reporting a slightly higher prevalence than men[iii]. Prevalence of lumbar spinal stenosis increases with age and body mass index[iv].
While the degree of stenosis on the imaging study is important, in clinical practice the clinician should correlate the clinical findings with the degree of stenosis evident on the imaging study. As with interpretation of all imaging studies, clinical correlation is important. In the study that supported FDA approval and the indications for use, moderate degenerative lumbar spinal stenosis, is defined as follows:
- 25% to 50% reduction in the central canal and/or nerve root canal (subarticular, neuroforaminal) compared to the adjacent levels on radiographic studies, with radiographic confirmation of any one of the following:
- Evidence of thecal sac and/or cauda equina compression
- Evidence of nerve root impingement (displacement or compression) by either osseous or non-osseous elements
- Evidence of hypertrophic facets with canal encroachment
AND associated with the following clinical signs:
- Presents with moderately impaired Physical Function (PF) defined as a score of ≥ 2.0 of the Zurich Claudication Questionnaire (ZCQ)
- Ability to sit for 50 minutes without pain and to walk 50 feet or more.
Clinical Presentation
Primary symptoms associated lumbar spinal stenosis include neurogenic claudication with back and leg pain, sensory loss, as well as weakness in the legs. More pronounced spinal stenosis may be presented through lower extremity weakness, muscle cramping, numbness and imbalance in gait. Symptoms are exacerbated by standing or walking, and extension of the spine. Short term relief may be observed when the patient is sitting or in flexion.
In addition to neurogenic claudication, lumbar spinal stenosis can present with symptoms that are more radicular in nature. As reported by Genevay and Atlas (2010)[v], “[u]nlike neurogenic claudication that is more commonly bilateral and associated with central canal stenosis, radicular symptoms due to spinal stenosis are more often unilateral and related to stenosis of the lateral recess or the foraminal canal. In these cases, patients tend to be younger and often have pain at rest and at night which is increased by the Valsalva maneuver. Leg pain is often described as severe and radicular in distribution and may be exacerbated with lumbar extension to the painful side (Kemp’s test)[vi]. Examination findings may include a limited lumbar range of motion especially in extension, focal motor weakness in a specific root distribution, variable straight-leg tension signs, and diminished subjective sensation and reflexes in specific root distributions”. In adults over the age of sixty, spondylosis (degenerative arthritis affecting the spine) is the most common cause of stenosis.
In their expert consensus on identifying the top six factors most important in the clinical diagnosis of lumbar spinal stenosis, Tomkins-Lane et al (2016)[vii] reported “leg or buttock pain while walking,” “flex forward to relieve symptoms,” “feel relief when using a shopping cart or bicycle,” “motor or sensory disturbance while walking,” “normal and symmetric foot pulses,” “lower extremity weakness,” and “low back pain.”
Poor Operative and Non-Operative Alternatives
Conservative options including physiotherapy, bracing, cane, opioid and non-opioid medications, and exercises are offered, but in practice, the lack of consistent and durable relief with these options decreases the usefulness for the patients afflicted with spinal stenosis. Even epidural injections, with or without steroids, though effective in some cases, are often precluded due to the dose of steroids that the patient can receive[viii]. In many cases, the epidural injections may provide temporary relief, but over the longer term, benefits of the therapy fade, leading the patients to seek surgical solutions. Cairns et al found persistent conservative care (>12 weeks) for lumbar spinal stenosis showed only minimal improvement in pain and function. Compared with extending conservative therapies or traditional spine surgery, interspinous lumbar decompression reduces both direct and indirect costs associated with lumbar spinal stenosis. Additionally, the costs of these conservative care options are not insignificant[ix]. Nonetheless, contemporary algorithms advocate for conservative care before indirect decompression systems [x]. Diwan et al (2019) recommend the use of minimally invasive indirect decompression systems which deliver indirect decompression for moderate lumbar spinal stenosis after a treatment of 6 months of conservative care. ILSIPP recommends conservative care for at least 6 months, the choice of what options for which should be individualized to the specific needs of the patients and be at the discretion of the treating physician.
The other extreme of the therapeutic spectrum viz open spinal surgery – with or without fusion – is reserved for those with severe spinal stenosis, cauda equina syndrome, instability, or severe scoliosis. This is because the benefits of surgery, even in the best-case scenarios is time limited, the perioperative morbidity and mortality are higher with open spinal surgeries, and the hospital stays and post-surgical rehabilitation requiring skilled nursing facility costs are greater with open spine surgeries[xi]. Published literature questions the benefits of complex fusion over simple laminectomy[xii]. Regardless of outcomes, the rates of simple decompression surgery and simple fusions have declined, while complex fusion surgery increased from 1.3 per 100,000 (just under 1% of operations) to 19.9 per 100,000 (14.6% of operations), a 15-fold increase (2002-2007). Adjusted mean hospital charges for complex fusion procedures were $80,888 compared to $23,724 for decompression alone[xiii]. Thus, there is a large unmet need, and a void in the therapeutic armamentarium[xiv].
Even if surgery was a credible option, in many cases the concurrent co-morbidities preclude surgical intervention due to the perioperative morbidity and mortality. In such cases, indirect decompression systems may be the only credible and durable option[xv].
Physician Qualification & Patient Selection
- Physician Qualifications
Implantation of indirect decompression systems without fusion should be performed only by qualified physicians, trained in the management of patients suffering from lumbar spinal stenosis and experienced in the placement of devices for whom patients would be indicated.
- Inadequate Response or Contraindicated for Conservative Therapies
Conservative care of no less than six months in duration should be provided to patients before implantation of indirect decompression systems. Physicians must be granted latitude based upon their clinical training, experience and what the physician and patient determine are best for the individual based upon circumstances unique to the individual. Conservative care may include, but is not limited to:
- Physical therapy for a duration of four to six weeks may be employed to reduce patient pain, disability and reliance of pain medication[xvi]. Studies report patients who have undergone physical therapy in advance of surgery furthermore enjoy faster recovery times[xvii].
- Modification in the patient’s activities of daily living may be considered. Sustainability and patient compliance, however, must be considered for each patient relative to other clinical and psychological needs, age, occupation, vocation, and geography.
- Oral medications including the use of non-narcotic analgesics and opiates may be prescribed. Physicians must use care and avoid negative drug-to-drug interactions and consider long-term effects of medications on other anatomical systems (e.g. longer-term use of NSAIDs and their association with gastrointestinal issues)[xviii]. Neuropathic medications are ineffective and often are poorly tolerated by the elderly affected with the condition, frequently leading to discontinuation[xix]. Thus, while the CDC 2016 guidelines mandates the use of non-opioid therapies or allows opioid doses of up to 90mg morphine equivalent[xx], these drugs are neither safe nor effective for the elderly patients afflicted with symptomatic neurogenic intermittent claudication pain spinal stenosis. Caution is warranted in the use of opioid therapy for the elderly, given the paucity of published evidence demonstrating efficacy for lumbar spinal stenosis in this population. Opiates are known to cause dependence, drowsiness, constipation (symptoms that are of particular concern in the elderly) and prove costly to the patient and health care system[xxi].
- Epidural steroid injections may be used to relieve neck, arm, leg and back pain caused by inflamed spinal nerves from lumbar spinal stenosis. ILSIPP published guidelines recommend the use of fluoroscopically-guided caudal epidural injections, as well as for fluoroscopically guided lumbar interlaminar epidural injections. The evidence for lumbar transforaminal epidural injections is Level IV to III with moderate recommendation with fluoroscopically guided lumbar transforaminal epidural injections for long-term improvement. The evidence for percutaneous adhesiolysis in lumbar spinal stenosis is based on relevant, moderate to high quality randomized controlled clinical trials, observational studies, and systematic reviews[xxii].
Patients refractory to conservative care, or those for whom conservative therapies are not indicated and who otherwise meet the indications for use of indirect decompression systems without fusion for moderate stenosis should be considered. Considering poor outcomes with the conservative options, both independent reviews and Health and Human Services (HHS) best practices report call for early interventional pain management evaluation and treatment of all pain patients, including the elderly afflicted with spinal stenosis[xxiii]. In the Joint ILSIPP-NANS 2019 fact sheet, ILSIPP reiterated its support of the 2019 HHS Best practices report that advocates for interventional pain therapies including indirect decompression systems.
- Radiographic Confirmation
Imaging studies are correlative with presentation of the patient’s signs and symptoms. Given their high sensitivity and specificity, magnetic resonance imaging (MRI) or computed tomography (CT) myelogram studies may be used to confirm diagnosis of lumbar spinal stenosis[xxiv].
- Informed Consent
Treatment options, risks associated with conservative care, epidural injections, opioid use and surgical options must be well understood by the patient when developing care plans for the individual. Physicians must ensure informed consent of the patient.
- Contraindications and Relative Contraindications
The use of indirect decompression systems are contraindicated for patients with severe osteoporosis, spondylolisthesis with dynamic instability (as defined by >5mm movement between flexion and extension roentgenograms, greater than Grade 1 spondylolisthesis, allergies to titanium or titanium alloy, cauda equina syndrome, scoliosis (Cobb angle >10 degrees) and morbid obesity defined as a body mass index >40. Active systemic and local infection are also contraindications.
Likewise, patients with previous open laminectomy and or fusion at the target level are considered to be inappropriate, but not patients who have had minimally invasive lumbar spinal decompression or other percutaneous procedures where the spinous process and the lamina are preserved.
Evidence-Based Rationale
Indirect decompression systems used to treat moderate lumbar spinal stenosis are implanted posteriorly using minimally invasive techniques without disruption to the osseous or ligamentous tissue. Implantation typically occurs within the hospital outpatient or ambulatory surgical center, using cannulas under fluoroscopic guidance. Contraindications for indirect decompression systems include patients at risk for spinous process fracture (e.g. severe osteoporosis), spondylolisthesis with dynamic instability >than Grade 1[xxv]. Allergies to titanium or titanium alloy, cauda equina syndrome, scoliosis (Cobb angle >10 degrees) and morbid obesity defined as a body mass index >40.
Mechanisms of action associated with indirect decompression systems of the spinal cord and nerve roots lead to immediate symptom relief[xxvi]. Cadaveric studies have shown increases in the spinal dimensions. For example, Falowski et al (2019)[xxvii] Table 1 details increases in canal and foraminal dimensions following implantation of an indirect decompression system.
Results from a prospective, randomized controlled clinical trial were published by Patel et al (2015)[xxviii]. This Level Ib evidence found the Superion indirect decompression system [Boston Scientific; Marlborough MA] relieved moderate lumbar spinal stenosis through two years post implant. Twenty-nine sites enrolled 391 patients, randomized to the index procedure or FDA approved control [X-STOP; Medtronic, Minneapolis MN]. At two years post implant, study subjects reported a 70% reduction in leg pain, 68% reduction in back pain, and clinical success measured by the Oswestry Disability Index (ODI) achieved in 65% of the patients. Superion success rates were reported as 99.5% for the index procedure, and 99.0% for the control.
There were no reported instances of device component fracture, disassembly or collapse. There was no device dislodgement for the index procedure, while 11.9% reported for control subjects. Use of the stand-alone indirect decompression system preserves treatment options and may obviate the need for decompressive laminectomy and or fusion in the majority of patients carefully selected and within the approved indications for use[xxix].
Long-term outcomes reported at five-years post implantation have demonstrated sustained and durable treatment effect. Nunley et al (2017)[xxx] reported 84% of patients demonstrated clinical success on at least two of three ZCQ domains. Individual ZCQ domain success rates were 75%, 81% and 90% for ZCQss, ZCQpf, and ZCQps, respectively. Leg and back pain success rates were 80% and 65%, respectively, and the success rate for ODI was 65%. Percentage improvements over baseline were 42%, 39%, 75%, 66%, and 58% for ZCQss, ZCQpf, leg and back pain VAS, and ODI, respectively (all P<0.001). Within-group effect sizes were classified as very large for four of five clinical outcomes (i.e., >1.0; all P<0.0001). Seventy-five percent of patients were free from reoperation, revision, or supplemental fixation at their index level at five years.
To collect real-world outcomes, a registry for patients treated with interspinous indirect decompression spacers for lumbar spinal stenosis with intermittent neurogenic claudication was conducted. Tekmeyster et al (2019)[xxxii] evaluated data from three-hundred sixteen physicians at 86 clinical sites located within the United States. Patient data were captured from in-person interviews and a phone survey. Outcomes included intraoperative blood loss, procedural time, leg and back pain severity (100 mm VAS), patient satisfaction and treatment approval at 3 weeks, 6 and 12 months. The mean age of registry patients was 73.0 ± 9.1 years of which 54% were female. Mean leg pain severity decreased from 76.6 ± 22.4 mm preoperatively to 30.4 ± 34.6 mm at 12 months, reflecting an overall 60% improvement. Corresponding responder rates were 64% (484 of 751), 72% (1,097 of 1,523) and 75% (317 of 423) at 3 weeks, 6 months and 12 months, respectively. Back pain severity improved from 76.8 ± 22.2 mm preoperatively to 39.9 ± 32.3 mm at 12 months (48% improvement); 12-month responder rate of 67% (297 of 441). For patient satisfaction at 3 weeks, 6 months and 12 months, 89%, 80%, and 80% were satisfied or somewhat satisfied with their treatment and 90%, 75%, and 75% would definitely or probably undergo the same treatment again. In the phone survey, the rate of revision was 3.6% (51 of 1,426).
For elderly patients suffering from significant comorbidities, implantation of indirect decompression systems were successfully shown to treat these patients at one or two levels. Doing so, Hartman et al (2019) reported avoidance of open spine surgery, anesthesia and risk of hospitalization commonly associated with this vulnerable patient population[xxxiii].
Diwan et al (2019) published their care algorithm based upon review of published evidence following inadequate response or failure of conservative care[xxxiv]. Researchers recommend the use of minimally invasive indirect decompression systems which deliver indirect decompression for moderate lumbar spinal stenosis. Implantation of these devices were supported due to long-term comparative trials and durability of treatment effect.
Cost Effectiveness
Using a Markov model evaluating cost-effectiveness of three treatment strategies for lumbar spinal stenosis, Parker et al (2015) concluded indirect decompression system implantation fell well below the QALY threshold of $50,000[xxxv] and that such intervention versus sustained conservative care provided superior value. Cairns et al (2019) found persistent conservative care (>12 weeks) for lumbar spinal stenosis showed only minimal improvement in pain and function. Compared with extending conservative therapies or traditional spine surgery, indirect decompression system reduces both direct and indirect costs associated with lumbar spinal stenosis[xxxvi].
Conclusions & Recommendations
The body of Level I-IV published evidence, long-term outcomes demonstrating durable treatment effect, avoidance of more invasive procedures and drug therapies, as well as consideration for the patient populations most likely to be candidates for indirect decompression systems should be considered within the standards of care for moderate lumbar spinal stenosis.
Policymakers and payers are strongly encouraged to enable timely access to FDA approved or cleared technologies, when deemed medically necessary and indicated for this procedure.
References
[i] Diwan et al. An Algorithmic Approach to Treating Lumbar Spinal Stenosis: An Evidenced-Based Approach. Pain Med. 2019 Dec 1;20(Suppl 2):S23-S31. doi: 10.1093/pm/pnz133
[ii] Lurie J, Tomkins-Lane C.. Management of lumbar spinal stenosis. BMJ 2016;352:h6234.
[iii] Parenteau, C.S., Lau, E.C., Campbell, I.C. et al. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci Rep 11, 5389 (2021).
[iv] Kalichman, L., Guermazi, A., Li, L. & Hunter, D. J. Association between age, sex, BMI and CT-evaluated spinal degeneration features. J. Back Musculoskelet. Rehabil. 22, 189–195. (2009); Kalichman, L., Kim, D. H., Li, L., Guermazi, A. & Hunter, D. J. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 10, 200–208. (2010).
[v] Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010 Apr;24(2):253-65. doi: 10.1016/j.berh.2009.11.001. PMID: 20227646; PMCID: PMC2841052.
[vi] Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine 2000;25(3):389–94.
[vii] Tomkins-Lane C, Melloh M, Lurie J, et al. ISSLS prize winner: Consensus on the clinical diagnosis of lumbar spinal stenosis: Results of an international Delphi study. Spine 2016;4115:1239–46.
[viii] Manchikanti L, Knezevic NN, Navani A, Christo PJ, Limerick G, Calodney AK, Grider J, Harned ME, Cintron L, Gharibo CG, Shah S, Nampiaparampil DE, Candido KD, Soin A, Kaye AD, Kosanovic R, Magee TR, Beall DP, Atluri S, Gupta M, Helm Ii S, Wargo BW, Diwan S, Aydin SM, Boswell MV, Haney BW, Albers SL, Latchaw R, Abd-Elsayed A, Conn A, Hansen H, Simopoulos TT, Swicegood JR, Bryce DA, Singh V, Abdi S, Bakshi S, Buenaventura RM, Cabaret JA, Jameson J, Jha S, Kaye AM, Pasupuleti R, Rajput K, Sanapati MR, Sehgal N, Trescot AM, Racz GB, Gupta S, Sharma ML, Grami V, Parr AT, Knezevic E, Datta S, Patel KG, Tracy DH, Cordner HJ, Snook LT, Benyamin RM, Hirsch JA. Epidural Interventions in the Management of Chronic Spinal Pain: American Society of Interventional Pain Physicians (ASIPP) Comprehensive Evidence-Based Guidelines. Pain Physician. 2021 Jan;24(S1):S27-S208. PMID: 33492918.
[ix] Cairns K et al. Cost-effectiveness and Safety of Interspinous Process Decompression (Superion)Pain Medicine, 20(S2), 2019, S2–S8 Review article
[x] Diwan et al. An Algorithmic Approach to Treating Lumbar Spinal Stenosis: An Evidenced-Based Approach. Pain Med. 2019 Dec 1;20(Suppl 2):S23-S31. doi: 10.1093/pm/pnz133).
[xi] Ghogawala Z, et al.: Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. NEJM 2016; Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis (2 years) N Engl J Med. 2008;358(8):794-810; Weinstein JN, et al.: Surgical versus Non-Operative Treatment for Lumbar Spinal Stenosis. Four-Year Results of the Spine Patient Outcomes Research Trial (SPORT). Spine 2010.
[xii] Forsth P et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016; 374:1413-1423).
[xiii] Deyo RA et al. Trends, Major Medical Complications, and Charges Associated with Surgery for Lumbar Spinal Stenosis in Older Adults. JAMA 2010; 303,1259-1265.
[xiv] Lauryssen C, et al.: Stand-alone interspinous spacer versus decompressive laminectomy for treatment of lumbar spinal stenosis. Expert Rev Med Devices 2015; 12(6):763-769.
[xv] Hartman J, Granville M, Jacobson RE. The Use of Vertiflex® Interspinous Spacer Device in Patients With Lumbar Spinal Stenosis and Concurrent Medical Comorbidities. Cureus. 2019 Aug 12;11(8):e5374. doi: 10.7759/cureus.5374. PMID: 31616607; PMCID: PMC6786837.
[xvi] Slater J, Kolber MJ, Schellhase KC, et al. The influence of exercise on perceived pain and disability in patients with lumbar spinal stenosis. A systematic review of randomized controlled trials. Am J Lifestyle Med 2016;102:136–47.
[xvii] Lindback Y, Tropp H, Enthoven P, Abbott A, Oberg B.. PREPARE: Presurgery physiotherapy for patients with degenerative lumbar spine disorder: A randomized controlled trial. Spine J 2018;188:1347–55.
[xviii] Kuritzky L, Samraj GP. Nonsteroidal anti-inflammatory drugs in the treatment of low back pain. J Pain Res. 2012;5:579-590. doi:10.2147/JPR.S6775.
[xix] Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol 2015; 14:162–73.
[xx] Dowell, D., Haegerich, T. M., & Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016 Morbidity and Mortality Weekly Report. JAMA – J Am Med Assoc 2016 at <http://www.cdc.gov/mmwr/cme/conted.html.
[xxi] Adogwa O, Davison MA, Vuong VD, et al.. Long term costs of maximum non-operative treatments in patients with symptomatic lumbar stenosis or spondylolisthesis that ultimately required surgery: A five-year cost analysis.
[xxii] Manchikanti L, Knezevic NN, Navani A, Christo PJ, Limerick G, Calodney AK, Grider J, Harned ME, Cintron L, Gharibo CG, Shah S, Nampiaparampil DE, Candido KD, Soin A, Kaye AD, Kosanovic R, Magee TR, Beall DP, Atluri S, Gupta M, Helm Ii S, Wargo BW, Diwan S, Aydin SM, Boswell MV, Haney BW, Albers SL, Latchaw R, Abd-Elsayed A, Conn A, Hansen H, Simopoulos TT, Swicegood JR, Bryce DA, Singh V, Abdi S, Bakshi S, Buenaventura RM, Cabaret JA, Jameson J, Jha S, Kaye AM, Pasupuleti R, Rajput K, Sanapati MR, Sehgal N, Trescot AM, Racz GB, Gupta S, Sharma ML, Grami V, Parr AT, Knezevic E, Datta S, Patel KG, Tracy DH, Cordner HJ, Snook LT, Benyamin RM, Hirsch JA. Epidural Interventions in the Management of Chronic Spinal Pain: American Society of Interventional Pain Physicians (ASIPP) Comprehensive Evidence-Based Guidelines. Pain Physician. 2021 Jan;24(S1):S27-S208. PMID: 33492918.
[xxiii] Bates D et al. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Medicine, 20, 2019, S2–S12; U.S. Department of Health and Human Services (2019, May). Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. Retrieved from U. S. Department of Health and Human Services website: https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html. ASIPP-NANS factsheet).
[xxiv] de Schepper EI, Overdevest GM, Suri P, et al. Diagnosis of lumbar spinal stenosis: An updated systematic review of the accuracy of diagnostic tests. Spine 2013;388:E469–81.
[xxv] Reference: Superion Interspinous Spacer, FDA PMA approval order and indications for use (P140004) accessed at:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P140004.
[xxvi] Block JE, Lavelle WF, Nunley PD. Toward a cure for lumbar spinal stenosis: The potential of interspinous process decompression. Med Hypotheses. 2019 Nov;132:109357. doi: 10.1016/j.mehy.2019.109357. Epub 2019 Aug 10. PMID: 31421414.
[xxvii] Falowski SM, Sayed D, Deer TR, Brescacin D, Liang K. Biomechanics and Mechanism of Action of Indirect Lumbar Decompression and the Evolution of a Stand-alone Spinous Process Spacer. Pain Med. 2019 Dec 1;20(Suppl 2):S14-S22. doi: 10.1093/pm/pnz129. PMID: 31808533; PMCID: PMC7101165.
[xxviii] Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, Miller LE, Block JE, Geisler FH. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine (Phila Pa 1976). 2015 Mar 1;40(5):275-82. doi: 10.1097/BRS.0000000000000735. PMID: 25494323.
[xxix] Lauryssen C, Jackson RJ, Baron JM, Tallarico RA, Lavelle WF, Deutsch H, Block JE, Geisler FH. Stand-alone interspinous spacer versus decompressive laminectomy for treatment of lumbar spinal stenosis. Expert Rev Med Devices. 2015;12(6):763-9. doi: 10.1586/17434440.2015.1100071. Epub 2015 Oct 21. PMID: 26487285.
[xxx] Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017 Sep 6;12:1409-1417. doi: 10.2147/CIA.S143503. PMID: 28919727; PMCID: PMC5593396.
[xxxi] Nunley PD, Deer TR, Benyamin RM, Staats PS, Block JE. Interspinous process decompression is associated with a reduction in opioid analgesia in patients with lumbar spinal stenosis. J Pain Res. 2018 Nov 20;11:2943-2948. doi: 10.2147/JPR.S182322. PMID: 30538533; PMCID: PMC6251434.
[xxxii] Tekmyster G, Sayed D, Cairns KD, Raso LJ, Kim C, Block JE. Interspinous Process Decompression With The Superion Spacer For Lumbar Spinal Stenosis: Real-World Experience From A Device Registry. Med Devices (Auckl). 2019 Oct 3;12:423-427. doi: 10.2147/MDER.S220431. PMID: 31632160; PMCID: PMC6781846.
[xxxiii] Hartman J, Granville M, Jacobson RE. The Use of Vertiflex® Interspinous Spacer Device in Patients With Lumbar Spinal Stenosis and Concurrent Medical Comorbidities. Cureus. 2019 Aug 12;11(8):e5374. doi: 10.7759/cureus.5374. PMID: 31616607; PMCID: PMC6786837.
[xxxiv] Diwan S, Sayed D, Deer TR, Salomons A, Liang K. An Algorithmic Approach to Treating Lumbar Spinal Stenosis: An Evidenced-Based Approach. Pain Med. 2019;20(Suppl 2):S23-S31. doi:10.1093/pm/pnz133.
[xxxv] Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: Conservative care, laminectomy, and the Superion interspinous spacer. Int J Spine Surg. 2015 Jul 9;9:28. doi: 10.14444/2028. PMID: 26273546; PMCID: PMC4528571.
[xxxvi] Cairns K, Deer T, Sayed D, van Noort K, Liang K. Cost-effectiveness and Safety of Interspinous Process Decompression (Superion). Pain Med. 2019 Dec 1;20(Suppl 2):S2-S8. doi: 10.1093/pm/pnz245. PMID: 31808529; PMCID: PMC6896024.
Position Statement on Spinal Cord Stimulation
for Patients with Painful Diabetic Neuropathy
The ILLINOIS SOCIETY OF INTERVENTIONAL PAIN PHYSICIANS supports the use of Senza®, Senza II™ and Senza Omnia™ Spinal Cord Stimulation (SCS) systems for diabetic neuropathy in patients refractory to conventional therapy.
Diabetic peripheral neuropathy is a common complication of diabetes presenting as pain and other dysesthesias, including numbness, burning, or tingling. Approximately 20% of patients with diabetes will develop painful diabetic neuropathy (PDN), a progressive, potentially debilitating chronic neuropathic pain condition which is described as burning, tingling, shooting, sharp and lancinating pain, initially starting in both feet, with the potential to progress up to the legs (often to the knees) and to the hands over time.1,2,3
Current PDN treatments include medications such as gabapentinoids, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, opioids, and topical solutions. Randomized clinical trials demonstrate limited efficacy of these medications with high incidence of adverse effects with a majority of patients discontinuing medications by 6 months.1,6,7
The clinical burden of diabetic neuropathy is well-documented, and studies have demonstrated increased morbidity and mortality related to ulcers, infections, amputations, pain management, and poor adherence to therapy. Compared to the general population, individuals with diabetic neuropathy are at an increased risk of chronic pain, foot ulcerations, foot infections, and amputations. A retrospective claims analysis over a five-year time horizon found amputation risk in the PDN subgroup was 16.24 times that of diabetic controls4
In addition to the clinical burden, PDN is associated with considerable direct and indirect economic costs and high healthcare resource utilization.4,5 In a retrospective claims analysis of healthcare utilization costs of patients with diabetes, direct medical costs were 4 times higher for patients with PDN vs patients with diabetes alone, $31,211 vs $7,875, respectively.4
There is an unmet need for this patient population and ILLINOIS SOCIETY OF INTERVENTIONAL PAIN PHYSICIANS support the use of the Senza® SCS systems as the only non-pharmacologic option, with a specific FDA-approved indication, for the treatment of pain associated with diabetic neuropathy in patients refractory to conventional therapy.
FDA Indication and Labeling
The Senza®, Senza II™, and Senza Omnia™ Spinal Cord Stimulation (SCS) Systems are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following: failed back surgery syndrome, intractable low back pain, and leg pain. Additionally, all Senza systems are indicated as aids in the management of chronic intractable pain of the lower limbs, including unilateral and bilateral pain associated with diabetic neuropathy.
The Senza system received CE mark in 2010, TGA approval in 2011, FDA PMA approval in 2015, and is commercially available in Europe, Australia, and the United States. In the U.S., Nevro’s Senza SCS systems are approved to deliver both traditional SCS low frequencies of 2 – 1,200 Hz and are the only systems approved to deliver 10,000 Hz frequencies. This combination of therapies offers the most waveform types in a single product, helping patients to achieve long-term pain relief.
Therapy Description
The Nevro SCS device portfolio includes Senza, Senza II, and Senza Omnia devices and are all minimally invasive, reversible, and typically prescribed for the treatment of pain of the back, trunk, and/or limbs. Stimulation at 10 kHz is unique to HFX™ and is indicated as paresthesia-independent therapy to provide pain relief without inducing paresthesias, a buzzing or tingling sensation common in lower frequency stimulation. This is especially well-matched for patients with PDN whose pain can already present with uncomfortable paresthesias.
Clinical Evidence
The SENZA-RCT was the first multicenter, prospective, randomized, controlled pivotal study of an SCS system with 24-month follow-up across 11 US pain centers comparing long-term results of 10 kHz therapy vs traditional low-frequency SCS. At both 12 and 24-month endpoints subjects in the 10 kHz study arm achieved statistically significant pain reduction and demonstrated long-term superiority of 10 kHz therapy compared with traditional SCS in both back and leg pain. Results of both the 12 and 24-month endpoints were published in the peer-reviewed scientific journals of Anesthesiology and Neurosurgery, respectively.8,9
The same mechanism of action demonstrated to be superior to traditional SCS in the SENZA-RCT is used to treat PDN. Rather than stimulating the dorsal column and creating paresthesias to mask the pain, 10 kHz SCS directly targets inhibitory pain-circuit neurons in the dorsal horn, providing pain relief without paresthesia.10
Painful Diabetic Neuropathy
On July 16, 2021, Nevro became the first and only company to receive FDA approval for an expanded indication for its Senza Spinal Cord Stimulation systems to treat patients with pain of the lower limbs associated with diabetic neuropathy.
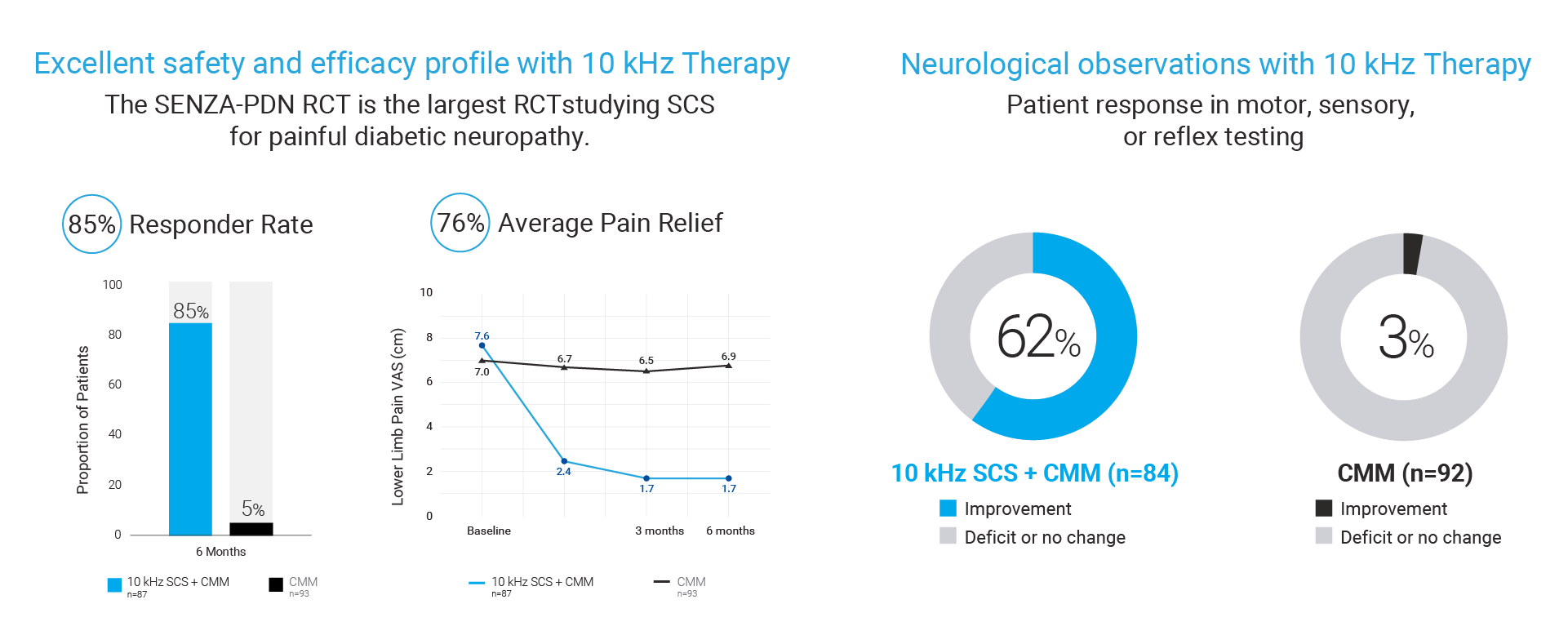
The FDA approval was based on an expansive body of clinical evidence including the largest RCT ever conducted for SCS in PDN patients showing the following results11:
- 85% of patients treated with 10 kHz Therapy achieved meaningful pain relief (as defined by 50% or more pain relief) vs only 5% of patients treated with conventional medical management (CMM).
- 76% average pain relief for 10 kHz Therapy patients vs -2% for patients with CMM alone
- 62% of 10 kHz Therapy patients reported neurological response such as reduced burning, numbness, tingling and cold sensation vs 3% for patients with CMM alone.
- 2% Explant Rate at 6-months with both explants due to infection and none due to loss of efficacy
References
- Pop-Busui R, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017 Jan;40(1):136-154.
- Gore M, et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374-385.
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350-354.
- Kiyani M, et al. Painful diabetic peripheral neuropathy: health care costs and complications from 2010 to 2015. Neurol Clin Pract. 2020 Feb;10(1):47-57.
- Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes. 2013;6:79-92.
- Gabapentin and risk of severe respiratory depression. Drug Ther Bull. 2018;56(1):3-4 doi: 10.1136/dtb.2018.1.0571
- YangM, et al. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16(11):2075-2083. doi:10.1111/pme.12845
- Kapural L, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT randomized controlled trial. Anesthesiology. 2015 Oct;123(4):851-60. doi: 0.1097/ALN.0000000000000774.
- Kapural L, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016 Nov;79(5):667-677. doi: 10.1227/NEU.0000000000001418.
- Lee, K, et al. Low-intensity, kilohertz frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience. 2020, 132-139. doi.org/10.1016/j.neuroscience.2019.12.031.
- Petersen, E, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy A Randomized Clinical Trial. JAMA Neurology, April 2021.
ILSIPP supports many clinical approaches to pain management, including neuromodulation. Access to these effective therapies is very important to ILSIPP members. As a medical society that represents many of the physicians performing these neuromodulation procedures, ILSIPP has not only the greatest interest but also has the greatest clinical expertise and experience to knowledgeably provide information and insight regarding these coding questions. As the opioid epidemic continues unabated, ILSIPP’s leadership on issues related to non-opioid pain treatments is critically important. The post here today is current as of 11/21/21 and updates that occur in the future may change or effect the accuracy of what is listed here. ILSIPP wants to make clear that ultimately billing and coding decisions are made on a case by case and are the sole responsibility of the physician who makes those decisions.
Following multiple inquiries from our membership, it is our understanding that there remains confusion regarding CPT codes 63685 (SCS) and 64590 (PNS) and their use with billing certain neurostimulation procedures and devices. These neurostimulation codes describe the creation of a distinct and separate pocket for a generator (implanted battery) or receiver (external battery).
ILSIPP has worked with leading members who perform these procedures, carefully reviewing the current CPT vignettes for these codes, and we confirm that the ‘physician work’ that is involved with creating a separate pocket for an IPG or receiver, as long as it is a distinct and separate pocket and incision, is consistent with the current language contained within the CPT vignettes that describe the surgical procedure for these two codes. ILSIPP notes that there are multiple new products and associated procedures available today. This applies to systems that are currently available for SCS and PNS neurostimulation system contain a separate receiver that must be assembled with the electrode array and implanted intra-operatively. For those currently available systems, the FDA-approved Information For Use (IFU) outlines and requires two distinct procedural incisions as well as creating a pocket to permanently anchor the receiver, thus preventing device migration. Our physician experts also confirm that additional work must be completed to assemble the multiple device components intra-operatively.
Therefore, it is the position of ILSIPP that CPT codes 63685 and 64590 represent the work performed by the physician and are appropriate for the physician to bill as it relates to the physician work involved in creating a receiver or generator pocket, as long as there is a distinct and separate incision, and a pocket is created for the neurostimulator.
Note: Lead insertions within the first incision are billed separately, using CPT codes 63650 (SCS) and 64555 (PNS).
| CPT Code | Code Description for Peripheral Nerve Stimulation |
| 64555 | Percutaneous implantation of neurostimulator electrode array, peripheral nerve (excludes sacral nerve) |
| 64590 | Insertion or replacement of peripheral or gastric neurostimulator pulse generator or receiver, direct or inductive coupling |
| CPT Code | Code description for Spinal Cord Stimulation |
| 63650 | Percutaneous implantation of neurostimulator electrode array, epidural |
| 63685 | Insertion or replacement of spinal neurostimulator pulse generator or receiver, direct or inductive coupling |
ILLINOIS SOCIETY OF INTERVENTIONAL PAIN PHYSICIANS
March Blog Post 2022
The ILLINOIS Society of Interventional Pain Physicians Position (ILSIPP) Statement in support of the use of :
Genicular Thermal Radiofrequency Ablation for Osteoarthritis of the Knee
In our continuing efforts to allow for patients to have improved quality of life with less pain via non addicting options the ILLINOIS Society of Interventional Pain Physicians supports the use of thermal radiofrequency ablation of the Genicular Nerves (also known as thermal ablation or denervation) for patients diagnosed with moderate to severe osteoarthritis of the knee. Patients afflicted with knee OA who may be refractory to conservative care including physical therapy, rest, ice/heat, OTC medications, surgical consultations, hyaluronic acid and corticosteroid injection who also do not have durable alternatives available to deliver pain relief and functional improvement beyond a few weeks to months1,2. Based upon the review of the body of peer-reviewed published evidence, approval by the United States Food and Drug Administration (FDA), real world experience and long-term patient outcomes, ILSIPP recommends qualified physicians consider use of this minimally invasive intervention and inform patients in their treatment plans based upon the clinical need and presentation. Because of proven safety and durable effectiveness, radiofrequency ablation of the genicular nerves of the knee using thermal RFA systems are within the clinical community’s standard of care for their indicated use. Based upon the body of Level I-IV peer-reviewed published evidence, FDA approval, and demonstration of cost-effectiveness, ILSIPP further recommends policymakers and payers enable timely access to thermal radiofrequency ablation of the genicular nerves for osteoarthritis of the knee when prescribed by a qualified physician who has used his or her best medical judgement for care most suitable to the individual diagnosed with moderate to severe knee osteoarthritis when this therapy is the best suited for the patient given their clinical presentation, when refractory to standard treatment, or when comorbidities preclude alternate treatment.
Knee Osteoarthritis: Burden of Disease
Although knee osteoarthritis, or degenerative joint disease of the knee, is closely correlated with age, it is typically the result of wear and tear and progressive loss of articular cartilage. It frequently manifests as a progressive disease leading to disability.3 Osteoarthritis of the knee is the most common type of arthritis affecting more than 30 million adults in the United States.4
Among the 18 million symptomatic OA knee pain sufferers, 6 million are between age 45- 65 and 2 million are under 45 years of age.5 A significant treatment gap exists for many of these people due to the low efficacy and short duration of current conservative therapies,
lack of patient access to safe and effective minimally invasive treatments and need for avoidance of surgery. 6
Clinical presentation, patient history along with radiographic confirmation of osteoarthritis, via x-ray, MRI, or CT, of grade 2 (mild), 3(moderate) or 4 (severe) in the past 12 months, pain of at least 6 out of 10 on a NRS is supported by the inclusion criteria of studies used to obtain FDA clearance for use of genicular radiofrequency ablation for knee OA pain
Clinical Presentation
The intensity of knee OA symptoms vary from individual to individual. The clinical symptoms of Knee OA include a chief complaint of knee pain:
- Typically of gradual onset
- Worse with prolonged activity, repetitive bending or stairs, inactivity
- Worsening over time
- Better with rest, ice, or anti-inflammatory medication
- Knee stiffness and swelling
- Decreased walking ability
Articular cartilage is composed of type II collagen, proteoglycans, chondrocytes, and water. In healthy joints, equilibrium between these components is maintained in that any degradation of cartilage is matched by synthesis. With osteoarthritis, the matrix metalloproteases, or degradative enzymes, are overexpressed resulting in loss of collagens and proteoglycans. 7
In the early stages of OA, chondrocytes secrete tissue inhibitors in an attempt to increase the synthesis of proteoglycans to overcome the degradation, but this reparative attempt is
not enough and eventually a loss of equilibrium results in loss of articular cartilage and elasticity, cracking and fissuring and erosion of the articular surface.
Primary knee osteoarthritis is the result of degenerative articular cartilage without any known reason. This may be due to age or wear and tear. Secondary knee osteoarthritis results from a known reason and can be due to trauma, surgery, limb malformation or malposition, chondrocalcinosis, infectious or psoriatic arthritis or other disease process.
Physical examination findings include visual inspection of the knee and may reveal periarticular erythema and swelling, quadriceps atrophy or varus/valgus deformity. Range of motion is an important finding with typical OA presenting as gross limitation of flexion and slight limitation of extension.
Low Efficacy & Durability of Non-Operative Alternatives
Medications aimed to treat inflammatory conditions may help, but they do not slow the progression and to date there are no disease modifying agents for the treatment of knee osteoarthritis.8 Non-surgical treatment for the pain and dysfunction of OA of the knee has
remain largely unchanged over the past 20 years.
Conservative treatment options begin with weight loss, physical therapy, opioid or non-opioid medications and typically progress to corticosteroid or hyaluronic acid injections with the definitive treatment for OA of the knee being total knee arthroplasty (TKA).
However, a significant treatment gap exists for millions of symptomatic OA knee pain sufferers due to low efficacy conservative therapies. The mean duration of their pain from onset is 10 years with 7 years of disability.9 Even when TKA is an appropriate option, factors including age, BMI or comorbidities are often considered and may preclude or necessitate deferment of surgery until more optimal medical conditions exist.
Intra-articular steroid (IAS) injection has long been a standard of care for the treatment of OA knee pain. However, IAS injections offer only short-term relief, often pose side effects, and lack strong evidence of efficacy. The reported duration of pain relief of intraarticular corticosteroid injections is one to two weeks with some trials demonstrating pain relief of up to three to four weeks. 10
Viscosupplementation with hyaluronic acid, or its derivatives, is injected into the affected knee as a multi- or single dose application to provide lubrication and shock absorption. While some studies suggest reduced pain through 26 weeks, 11 the FDA has questioned its mechanism of action and current clinical practice guidelines do not support use of HA for the treatment of OA knee pain.12,13
Neither opioids, IAS nor HA are recommended by the American Academy of Orthopedic Surgeons (AAOS) for the treatment of knee osteoarthritis in their Clinical Practice Guidelines.14
Radiofrequency ablation (RFA) was first described in the literature for treating chronic back pain in 1975.15 Radiofrequency is a minimally invasive, non-surgical outpatient procedure used to target the genicular nerves of the knee causing chronic pain. The procedure involves minimal sedation and typically is completed in less than 45 minutes using a RF generator to transmit a small current of RF thermal energy through an insulated electrode placed within the tissue. Radiofrequency ablation delivers targeted thermal damage in excess of 43˚ C to neuronal tissue in order to modulate transmission of pain signals using a simple electrode structure that generates RF energy.16 While genicular nerve course variability is high, the consensus and data supports clinical effect from targeting the following three primary nerve targets: the superomedial and inferomedial branches of the saphenous nerve and the superolateral branch of the femoral nerve.17,18
Radiofrequency of the genicular nerves can address the treatment gap between conservative therapy and TKA by offering a clinically superior and more durable option when patients are refractory to current standard care. Studies show that radiofrequency of the genicular nerves can reduce pain and improve function for at least 6-12 months.
With a projected 401% growth in demand for TKA’s over the next twenty years,19 thermal radiofrequency for OA of the knee can combat this escalating trajectory by deferring surgery for younger patients, of which 40% are performed on patients under age 65,20 and reduce the lifetime risk and cost associated with TKA revisions.
Prior to genicular thermal radiofrequency ablation, OHSIPP recommends conservative care for at least 3-6 months, the choice of what options for which should be individualized to the specific needs of the patients and be at the discretion of the treating physician.
Physician Qualification & Patient Selection
- Physician Qualifications
Thermal radiofrequency ablation should be performed only by qualified physicians, trained in the management of patients suffering from chronic pain f osteoarthritis and experienced in the application of radiofrequency ablation and nerve denervation in patients for which the procedure is appropriately indicated. - Inadequate Response or Contraindicated for Conservative Therapies
Thermal radiofrequency ablation is intended for creating radiofrequency lesions of the genicular nerves for the management of moderate to severe knee pain of more than 6 months in patients with radiologically-confirmed osteoarthritis (grade 2-4) and a positive response (≥50% reduction in pain) to a diagnostic genicular nerve block. Select candidates may have undergone prior conservative therapy, including medication. Prior treatments received may include physical therapy, weight loss, exercise, NSAIDs, opioids, and intraarticular steroid or hyaluronic acid injections. - Physical therapy for a duration of four to six weeks may be employed to reduce patient pain, disability, and reliance of pain medication
- Modification in the patient’s activities of daily living may be considered. Sustainability and patient compliance, however, must be considered for each patient relative to other clinical and psychological needs, age, occupation, vocation, and
geography. - Oral medications including the use of non-narcotic analgesics and opiates may be prescribed. NSAIDS, while commonly utilized to help with pain management, are not without risks that include gastrointestinal, cardiovascular, renal, and respiratory adverse effects.21
- Caution is warranted in the use of opioid therapy, particularly for the elderly. Opioid drugs have become a widespread non-surgical intervention for chronic musculoskeletal conditions. An estimated 20% of patients presenting to the physician office with non-cancer pain symptoms or pain-related diagnoses receive an opioid prescription.22 In addition to physical side effects such as drowsiness and constipation, opiates are known to cause dependence with as many as one in five patients becoming a routine user after 10 days of narcotic analgesia. 23
Patients with imaging studies correlative with presentation of the patient’s signs and symptoms, refractory to conservative care, or those for whom conservative therapies are not indicated and who otherwise meet the indications for thermal radiofrequency of the genicular nerves should be considered as well as those medically incapable or unwilling to undergo surgical intervention.
- Informed Consent
Treatment options, risks associated with conservative care, injections, opioid use, and surgical options must be well understood by the patient when developing care plans for the individual. Physicians must ensure informed consent of the patient. - Contraindications and Relative Contraindications
- For patients with cardiac pacemakers, a variety of changes can occur during and after the treatment. In sensing mode the pacemaker may interpret the RF signal as a heartbeat and may fail to pace the heart. Contact the pacemaker company to determine if the pacemaker should be converted to a fixed-rate pacing during the radiofrequency procedure. Evaluate the patient’s pacing system after the procedure.
- Check the compatibility and safety of combinations of other physiological monitoring and electrical apparatus to be used on the patient in addition to the RF Generator.
- If the patient has a spinal cord, deep brain, or other stimulator, contact the manufacturer to determine if the stimulator needs to be in the bipolar stimulation mode or in the OFF position.
- This procedure should be reconsidered in patients with any prior neurological deficit.
- The use of general anesthesia is contraindicated. To allow for patient feedback and response during the procedure, it should be performed under local anesthesia.
- Systemic infection or local infection in area of the procedure.
- Blood coagulation disorders or anticoagulant use.
Evidence-Based Rationale
Results of a prospective RCT of thermal radiofrequency ablation of the genicular nerves using water-cooled probes (CRFA) compared to hyaluronic acid injection (HA) demonstrates greater improvement in NRS pain scores and WOMAC functional ability in the CRFA group vs the HA group at 6-months and at 12-month follow-up. The mean NRS at 6 months was 2.7±2.3 for the CRFA group and 4.5±2.7 for the HA group (p<0.0001). The mean WOMAC score improvement at 6 months from baseline was 48 % in the CRFA group and 23% in the HA group (p<0.0001). At 6-months 72% of the CRFA subjects reported improvement in Global Perceived Effect compared to 40% in the HA group (p<0.0001). 24 At 12-months, 65.2% of the CRFA cohort reported ≥ 50% pain relief from baseline. Mean NRS was 2.8±2.4 at 12 months (baseline 6.9±0.8), representing a 4.1% decrease in NRS pain score (p<0.0001). Subjects in the CRFA cohort had a 46.2% improvement in total WOMAC score at the 12-month timepoint.25
Similarly, another prospective RCT compared thermal ablation of the genicular nerves using water-cooled probes (CRFA) to intraarticular steroid injection (IAS). At both 6 and 12 months, the CRFA group demonstrated greater pain relief and improved function as measured by NRS and Oxford Knee Score (OKS). At 6 months, the mean NRS was 2.5 ± 2.3 for the CRFA group and 5.9 ± 2.2 for the IAS group (p < 0.0001), representing a 4.9 -point drop in NRS for the CRFA group. The mean OKS was 35.7 ± 8.8 in the CRFA group at 6 months compared to 22.4 ± 8.5 in the IAS group (p < 0.0001). At 6 months, 91.4% of subjects in the CRFA group reported improvement in Global Perceived Effect compared to 23.9% in the IAS group (p < 0.0001). 26 At 12 months, 65% of the original CRFA group had pain reduction ≥50%, as measured by the NRS, and the mean overall drop was 4.3 points (p<0.0001). Seventy-five per cent reported “improved” effects. The OKS increase from baseline in the original CRFA cohort was 17.3±12 points (N=52, p<0.0001, Student’s paired t-test), with an absolute mean of 34.3±11.1 points. 27
A comparison study of thermal radiofrequency (RFA) of the genicular nerves to conventional analgesics demonstrated statistically significant improvement in VAS for RFA patients over analgesics at 3 (2.83±0.5 vs 4.93±0.2) and 6 months (3.13±0.3 vs 5.73±0.26). The total WOMAC index showed significant difference between the two groups by the 6th month only; however, WOMAC domains (pain, and stiffness) showed significant differences in the 3rd, and 6th months, with lower values in the RFA group.28
A trial of thermal radiofrequency (RFA) compared to hyaluronic acid (HA) demonstrated statistically significant improvement in VAS and Lysholm Knee Score (LKS) scores at 3, 6, 9, and 12 months. 29 When compared to sham, thermal genicular radiofrequency (RFA) under fluoroscopic guidance showed VAS scores in the RFA group to be less at 4 (p< 0.001) and 12 (p < 0.001) weeks compared with the control group. Oxford Knee Scores showed similar findings (p < 0.001).30 In their Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hip, Hand and Knee, The American College of Rheumatology states that “radiofrequency ablation is conditionally recommended for patients with OA of the hand, hip and knee. 31
The American Society of Pain and Neuroscience recommends that “genicular nerve radiofrequency neurotomy may be used for the treatment of osteoarthritis related and post-surgical knee joint pain with a GRADE of II-1B.32
Cost Effectiveness
A cost-effectiveness analysis based on trial data evaluated the costs and health outcomes of patients undergoing cooled thermal radiofrequency ablation (CRFA) of the genicular nerves for OA knee pain compared to intraarticular steroid injections (IAS). The economic model calculated health benefits in the form of QALYs by mapping trial-based changes in function as measured using the Oxford Knee Score (OKS). The OKS captures patients’ assessment of knee symptoms and function. Costs were derived from the Centers for Medicare and Medicaid Services fee schedules and included standard physician (in-office or in-hospital) and hospital payments for IAS, CRFA and genicular nerve block procedures. At a time-horizon of 6 months post-treatment, CRFA was associated with a 0.091 gain in QALYs and an incremental cost effectiveness ration (ICER)of US$1711 compared with IAS. CRFA therefore cost US$18,773 per QALY gained.
Conclusions & Recommendations
The body of Level I-IV published evidence, long-term outcomes demonstrating durable treatment effect, avoidance of more invasive procedures and drug therapies, as well as consideration for the patient populations most likely to be candidates for thermal radiofrequency ablation of the genicular nerves should be considered within the standards of care for osteoarthritis related and post-surgical knee pain.
Policymakers and payers are strongly encouraged to enable timely access to FDA cleared technologies, when deemed medically necessary and indicated for this procedure.
Technology Definitions of Thermal Radiofrequency
Standard Radiofrequency (RFA) is a thermal ablation procedure. Treatment protocols typically apply 90 seconds of heating with a target temperature of 80 -90˚C. While nerve tissue begins to degrade at temperatures greater than 43˚ C, the 80˚ C protocol was derived from in vitro studies and is thought to be related to the highest temperature that early RF generators could attain.33
Water-Cooled Radiofrequency (CRFA) is a thermal ablation procedure offering physicians an alternate probe choice. Circulated water helps to carry heat away from the electrode-tissue interface to reduce charring allowing more energy to be delivered to surrounding tissues creating a larger area for ionic heating to occur. When using water- cooled probes studies have demonstrated the tissue temperature beyond the electrode tissue interface reach 80˚ C and greater and that the lesions created are larger than those of standard RF.34
References
- Cheng OT, Souzdalnitski D, Vrooman B, et al. Evidence-based knee injections for the management of arthritis. Pain Med. 2012; 13(6):740-753.
- Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Bailleul F, Pavelka K. Single, intra- articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomized, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010 Jan;69(1):113-9.
- Hsu H, Siwiec RM. Osteoarthritis, Knee. [Updated 2018 Jun 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507884/. Accessed Mar 4, 2022.
- Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res (Hoboken). 2016; 68(5):574–580. abstract
- Deshpande B, Katz J, Solomon D, et al. The number of persons with symptomatic knee osteoarthritis in the United States: Impact of race/ethnicity, age, sex and obesity. Arthritis Care Res (Hoboken). 2016 December; 68(12): 1743–1750. doi:10.1002/acr.22897.
- London N, Miller L, Block J. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Medical Hypotheses 76 (2011) 887–892
- Hsu H, Siwiec RM. Osteoarthritis, Knee. [Updated 2018 Jun 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507884/. Accessed Mar 4, 2022.
- Hsu H, Siwiec RM. Osteoarthritis, Knee. [Updated 2018 Jun 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507884/. Accessed Mar 4, 2022.
- OA Pain Landscape & Patient Journey; Research Results, KS&R, 2015.
- Cheng OT, Souzdalnitski D, Vrooman B, et al. Evidence-based knee injections for the management of arthritis. Pain Med. 2012; 13(6):740-753.
- Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Bailleul F, Pavelka K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomized, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010 Jan;69(1):113-9.
- FDA Intent to Consider the Appropriate Classification of Hyaluronic Acid Intra-Articular Products Intended for the Treatment of Pain in Osteoarthritis of the Knee Based on Scientific Evidence. Docket No. FDA-2018-N-4627.
- Jevsevar DS. (2013). Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition. J Am Acad Orthop Surg. 2013; 21:571-6.
- American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2013. www.aaos.org/Research/guidelines/TreatmentofOsteoarthritis oftheKneeGuideline.pdf.
- Shealy CN, Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg., 43(4) 1975, pp. 448-451.
- Kapural L, Deering J. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag.2020. 10(3):133-140
- Franco CD, et al. Innervation of the Anterior Capsule of the Human Knee: Implications for Radiofrequency Ablation. Regional Anesthesia and Pain Medicine 2015; 40:363-368
- Tran J, Peng P, Lam K, Baig E, Agur A, Gofeld M. Anatomical Study of the Innervation of Anterior Knee Joint Capsule Implication for Image-Guided Intervention. Regional Anesthesia and Pain Medicine. Volume 43, Number 4, May 2018
- Singh J, Yu S, Chen L, Cleveland J. Rates of total joint replacement in the United States: Future projections to 2020-2040 using the national inpatient sample. Jrhem 2019(47)2; DOI: https://doi.org/10.3899/jrheum.170990
- Losina E, Paltiel D, Weinstein A et al. Lifetime medical costs of knee osteoarthritis management in the United States: Impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015 February; 67(2): 203–215. doi:10.1002/acr.22412
- Solomon D., Nonselective NDAIDs: Overview of adverse effects. UpToDate. Accessed 5/10/2019 https://www.uptodate.com/contents/nonselective-nsaids-overview-of-adverse-effects
- Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care 2013; 51:870–8. CrossRef PubMed
- Centers for Disease Control and Prevention (CDC). Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6610a1.htm.Accessed Mar 7, 2022.
- Chen A, Khalouf F, Zora K et al. Cooled radiofrequency ablation compared with a single injection of hyaluronic acid for chronic knee pain. A multi-center, randomized, clinical trial demonstrating greater efficacy and equivalent safety for cooled radiofrequency ablation. J Bone Joint Surg. 2020; 102(17):1501-1510. doi:10.2106/JBJS.19.00935
- Chen A, Khalouf F, Zora K et al. Cooled radiofrequency ablation provides extended clinical utility in the management of knee osteoarthritis: 12-month results from a prospective, multi-center, randomized, cross-over trial comparing cooled radiofrequency ablation to a single hyaluronic acid injection. BMC Musculoskeletal Disorders (2020) 21:363. https://doi.org/10.1186/s12891-020-03380-5
- Davis T, Loudermilk E, DePalma M, Hunter C, Lindley D, Patel N, Choi D, Soloman M, Gupta A, Desai M, Buvanendran A, Kapural L. Prospective, Multicenter, Randomized, Crossover Clinical Trial Comparing the Safety and Effectiveness of Cooled Radiofrequency Ablation with Corticosteroid Injection in the Management of Knee Pain from Osteoarthritis. Reg Anesth Pain Med. 2018; 43(1):84-91.
- Davis T, Loudermilk E, DePalma M, Hunter C, Lindley D, Patel N, Choi D, Soloman M, Gupta A, Desai M, Cook E, Kapural L. Twelve-month analgesia and rescue, by cooled radiofrequency ablation treatment of osteoarthritic knee pain: results from a prospective, multi-center, randomized, cross-over trial. Reg Anesth Pain Med 2019;(0):1–8. DOI:10.1136/rapm-2018-100051. PMID: 30772821
- El Hakeim E et al. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: A single-blind randomized controlled trial. Pain Physician 2018; 21:169-177 • ISSN 1533-3159
- Xiao l et al. Highly selective peripheral nerve radio frequency ablation for the treatment of severe knee osteoarthritis. Experimental and Therapeutic Medicine 16: 3973-3977.2018
- Choi et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: A double-blind randomized controlled trial. Pain. 2011 Mar;152(3):481-487. doi: 10.1016/j.pain.2010.09.029. Epub 2010 Nov 4. PMID:21055873.
- Kolasinski S, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arth Care & Res. 72(2). 2000: 149-162
- Lee D et al. Latest Evidence-Based Application for Radiofrequency Neurotomy (LEARN): Best Practice Guidelines from the American Society of Pain and Neuroscience (ASPN). J of Pain Res. 2021:14 2807-2831
- Ball, R., The science of conventional and water-cooled monopolar lumbar radiofrequency rhizotomy; an electrical engineering point of view. Pain Physician 2014; 17: E175-211.
- Cedeno DL, Vallejo A, Kelley CA, Tilley DM, Kumar N. Comparisons of lesion volumes and shapes produced by a radiofrequency ˜ system with a cooled, a protruding, or a monopolar probe. Pain Physician 8, E915–E922 (2017).